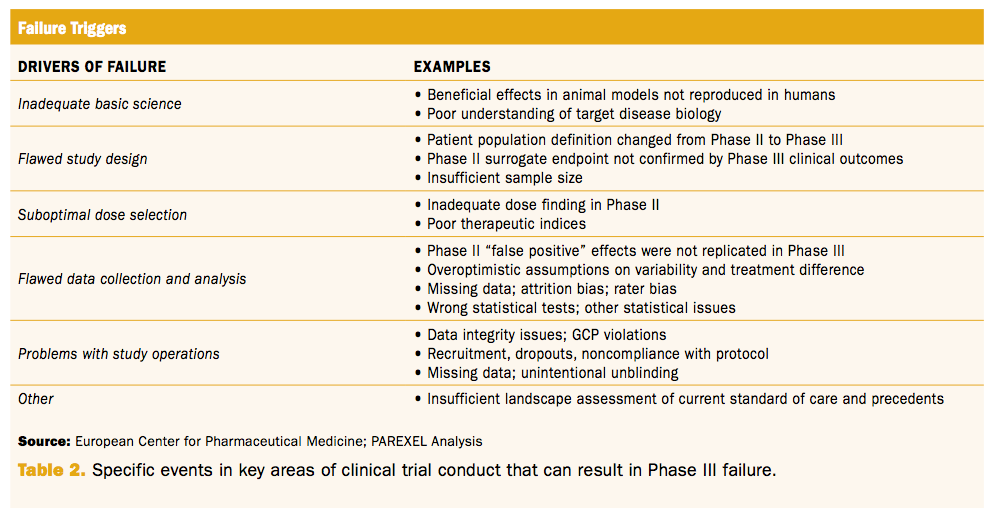
Quality of missing data reporting and handling in palliative care trials demonstrates that further development of the CONSORT statement is required: a systematic review - ScienceDirect

Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring - ESMO Open

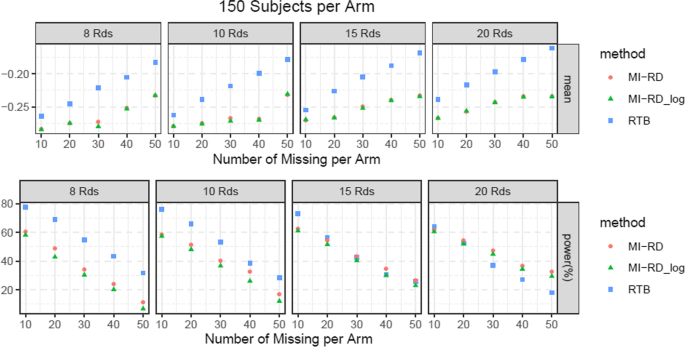
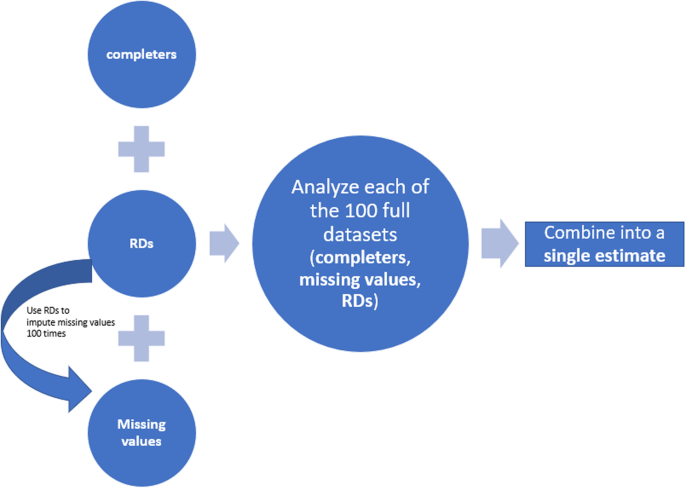
Missing Data Sensitivity Analysis of a Continuous Endpoint An Example from a Recent Submission - PDF Free Download

Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide - Cro - 2020 - Statistics in Medicine - Wiley Online Library

Quality of missing data reporting and handling in palliative care trials demonstrates that further development of the CONSORT statement is required: a systematic review - Journal of Clinical Epidemiology

Standards should be applied in the prevention and handling of missing data for patient-centered outcomes research: a systematic review and expert consensus - Journal of Clinical Epidemiology

An Empirical Comparison of Statistical Methods for Missing Data in Randomized, Double-Blind, Placebo-Controlled, Phase 3 Clinical Trials for Chronic Pain and Lipid-Lowering Products | SpringerLink

Guideline on Missing Data in Confirmatory Clinical Trials / guideline-on- missing-data-in-confirmatory-clinical-trials.pdf / PDF4PRO

Empirical evaluation of the implementation of the EMA guideline on missing data in confirmatory clinical trials: Specification of mixed models for longitudinal data in study protocols - Häckl - 2019 - Pharmaceutical
PDF) Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide

Sensitivity Analysis for Not-at-Random Missing Data in Trial-Based Cost-Effectiveness Analysis: A Tutorial | SpringerLink
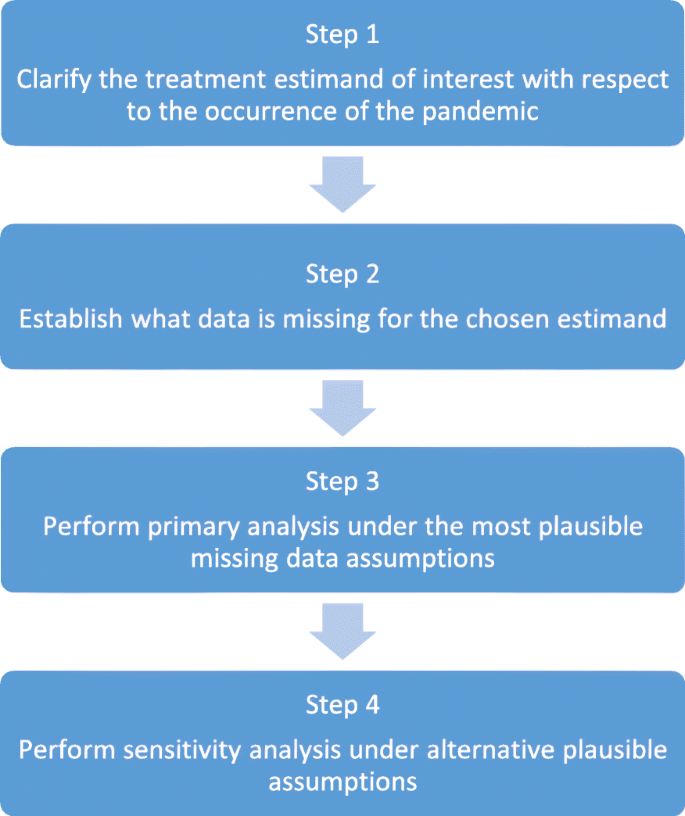
A four-step strategy for handling missing outcome data in randomised trials affected by a pandemic | BMC Medical Research Methodology | Full Text

Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models - Ratitch - 2013 - Pharmaceutical Statistics - Wiley Online Library

Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: A practical guide - Cro - 2020 - Statistics in Medicine - Wiley Online Library

Empirical evaluation of the implementation of the EMA guideline on missing data in confirmatory clinical trials: Specification of mixed models for longitudinal data in study protocols - Häckl - 2019 - Pharmaceutical
Reading: The Prevention and Treatment of Missing Data in Clinical Trials |The National Academies Press
A narrative review of estimands in drug development and regulatory evaluation: old wine in new barrels? | Trials | Full Text