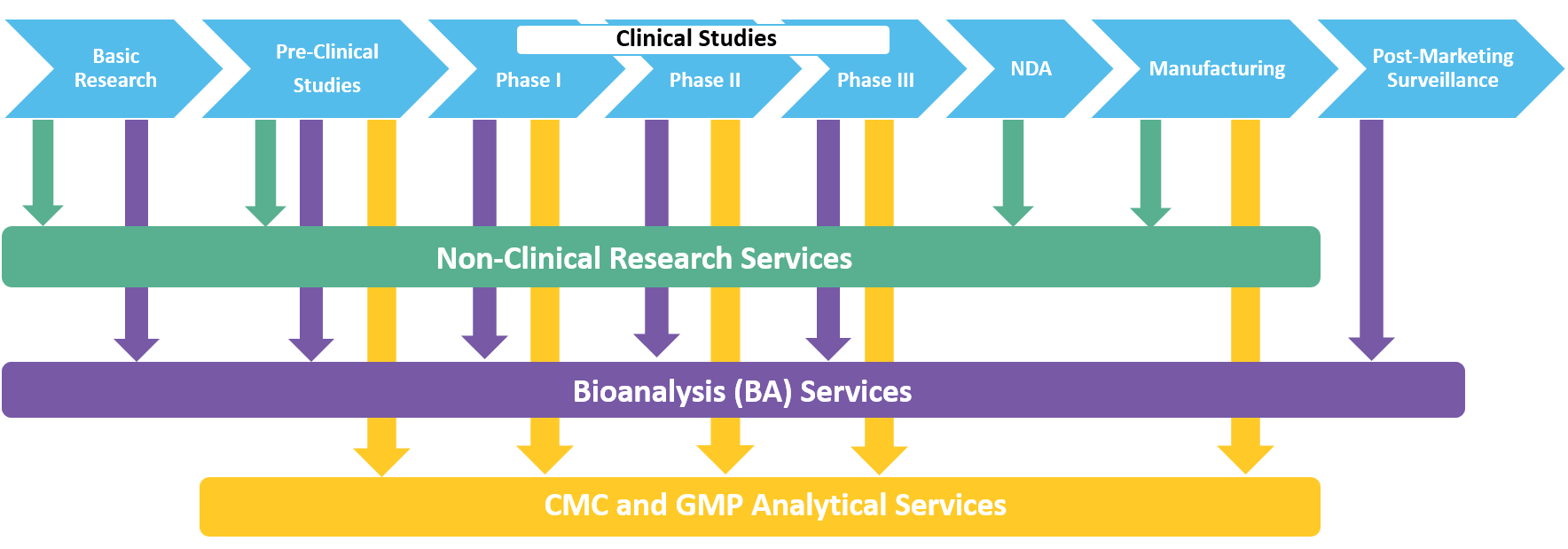
Regulatory Compliance in Pharmaceutical Development: GLP & GMP Jeffrey G. Sarver, Ph.D. MBC 3100 March 8, questions to: - ppt video online download

Early Development GMPs for Drug-Product Manufacturing of Small Molecules: An Industry Perspective (Part III)
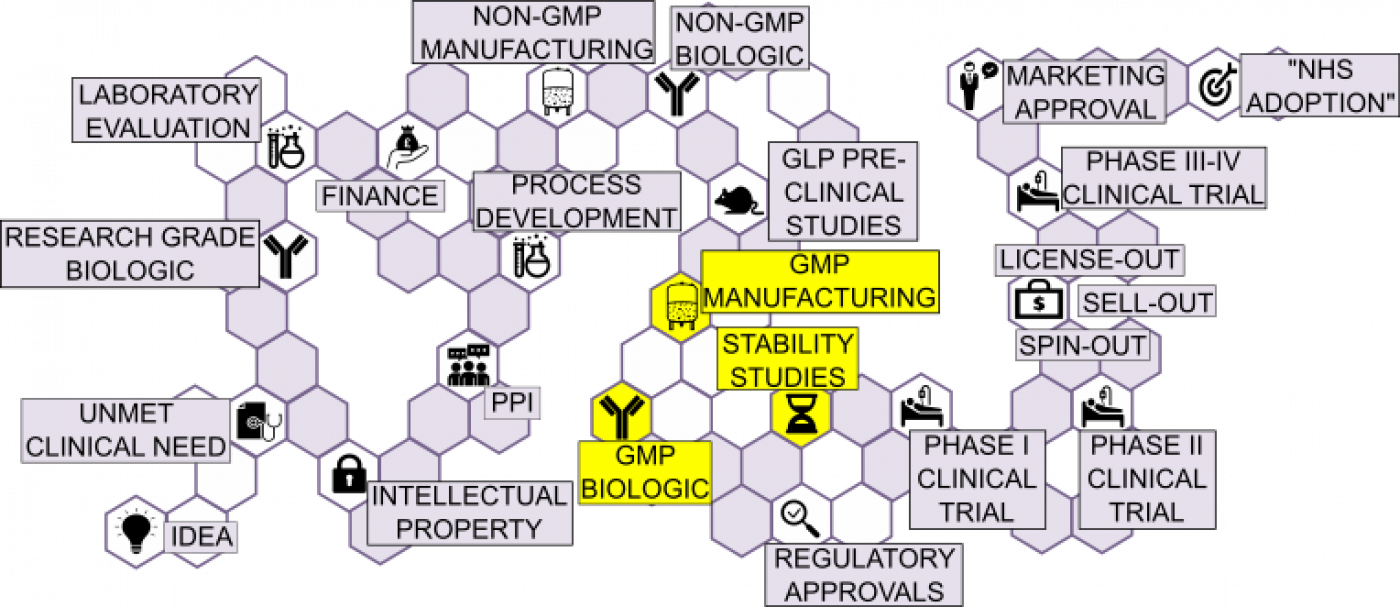
Manufacturing a Clinical Trial Product (Biologics) | UCL Therapeutic Innovation Networks - UCL – University College London

Phases of alcohol drug development. GMP = Good Manufacturing Practice;... | Download Scientific Diagram
Investigator Initiated Trials (IIT) – Considerations and Guidance from the Perspective of Clinical Trial Supplies and GMP | ISPE | International Society for Pharmaceutical Engineering












![The Quality System in the pharmaceutical context [Grandi Strumentazioni e Core Facilities (FAST)] The Quality System in the pharmaceutical context [Grandi Strumentazioni e Core Facilities (FAST)]](https://corefacilities.iss.it/dw/lib/exe/fetch.php?media=en:aree:fabiocell:sq.png)