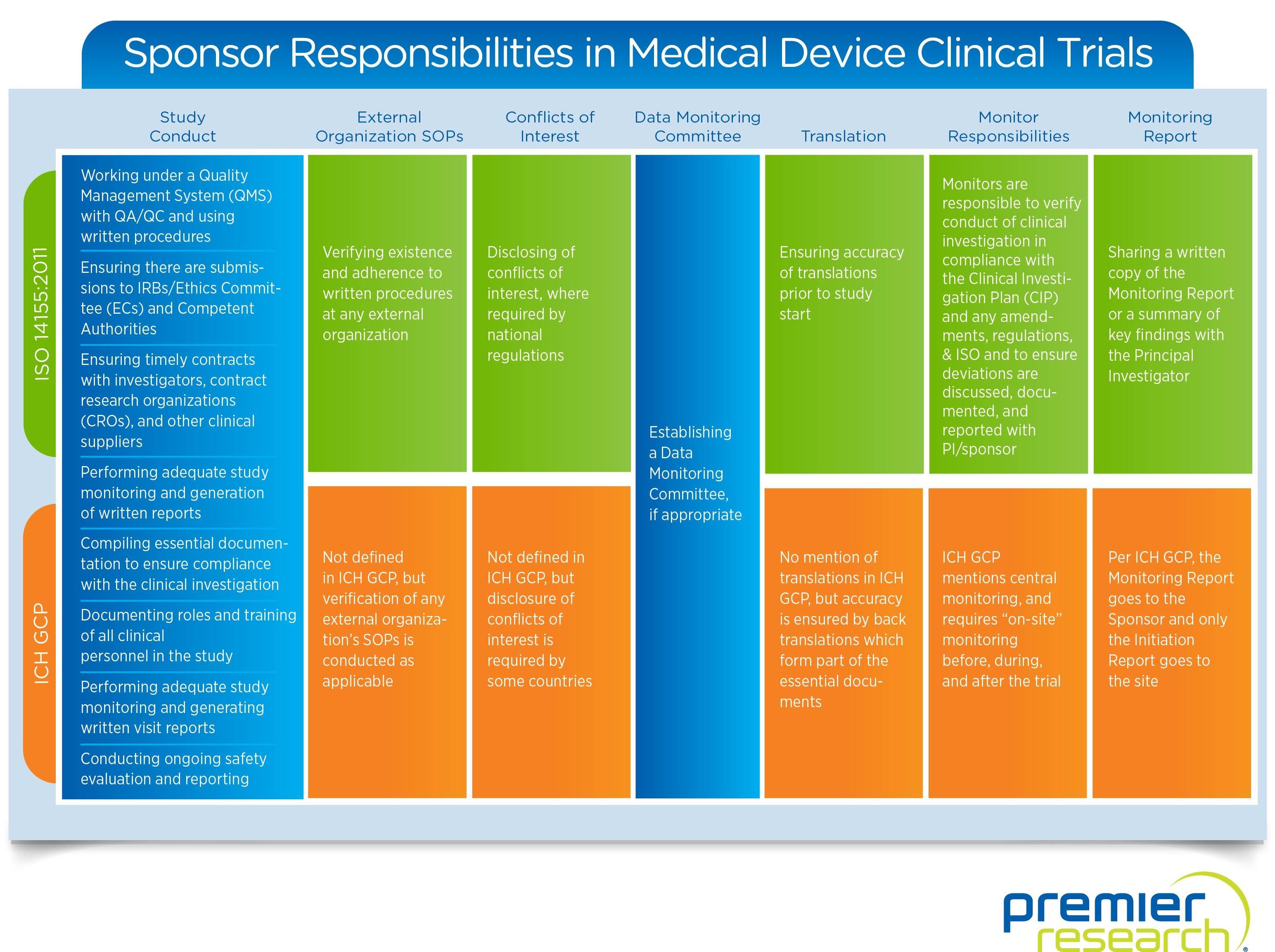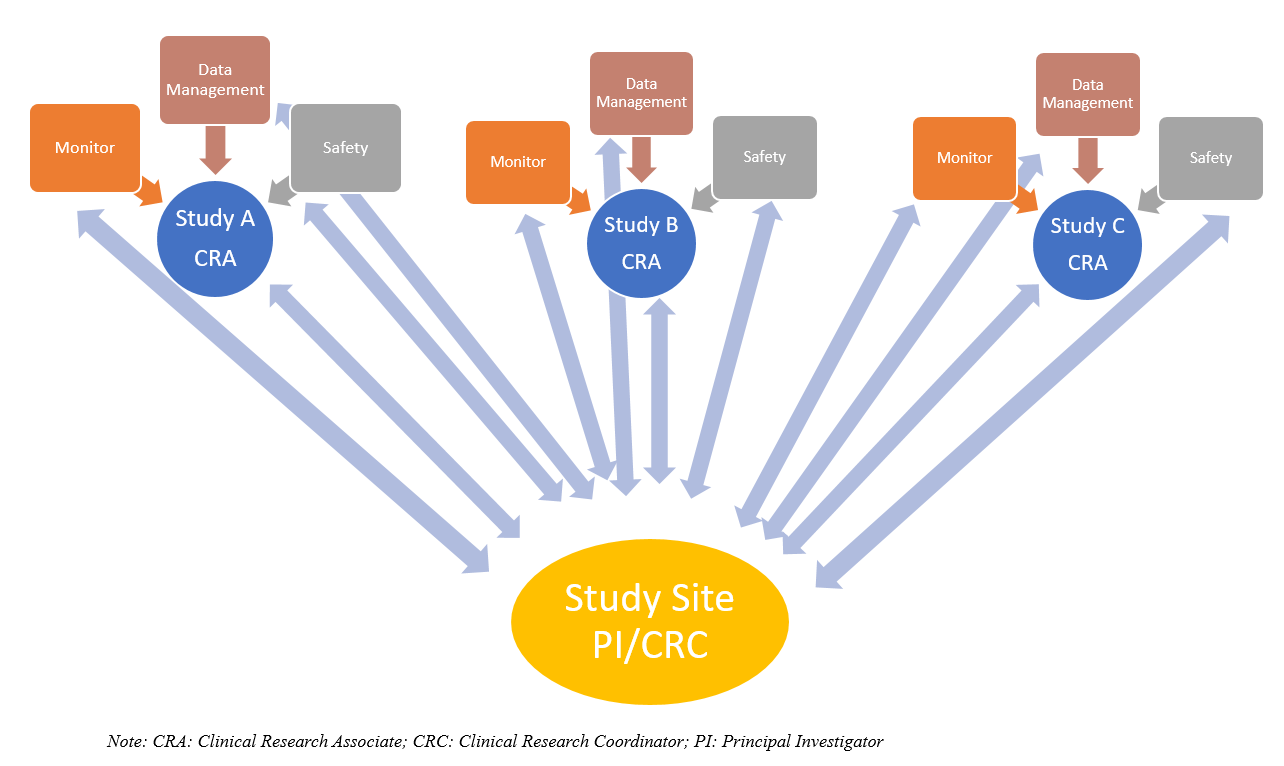
Sponsor-Site Communication in Device Trials: Evolution of a Dedicated Field Clinical Organization Throughout Study Execution - ACRP

The Good Clinical Practice (GCP) and the responsibilities of pharma sponsors - Avantyo article in Viata Medicala magazine · News · Avantyo
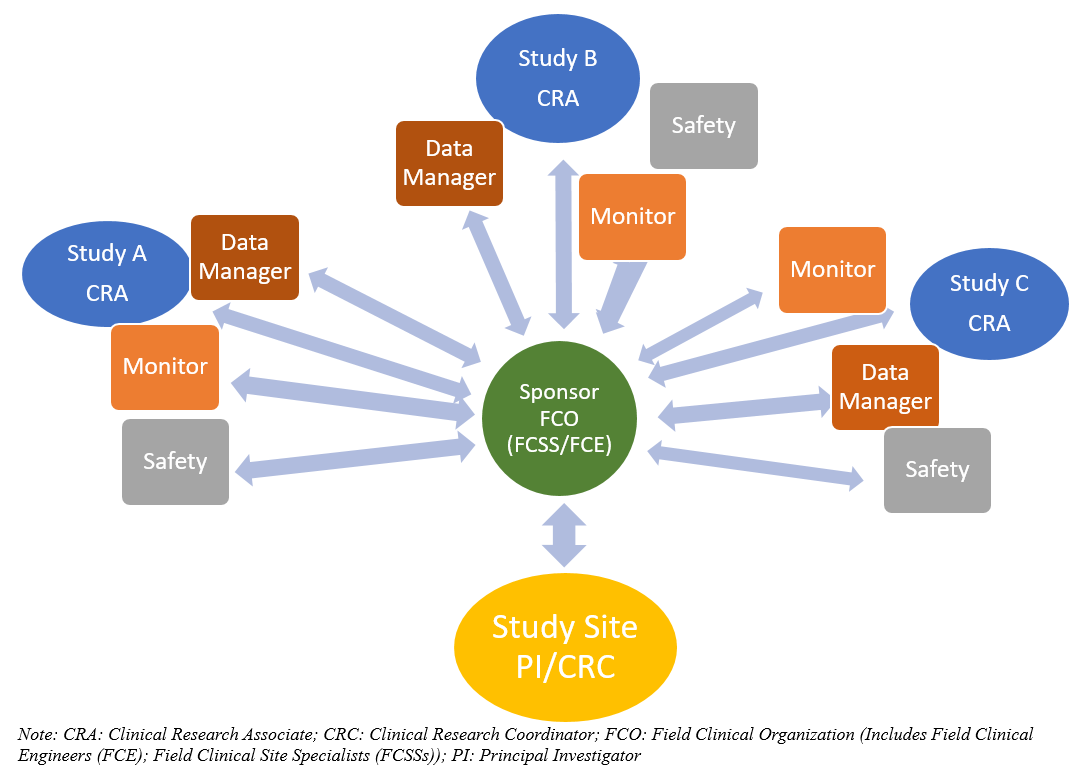
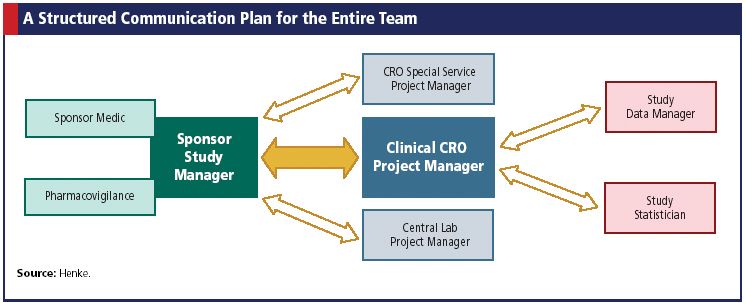
Sponsor-Site Communication in Device Trials: Evolution of a Dedicated Field Clinical Organization Throughout Study Execution - ACRP

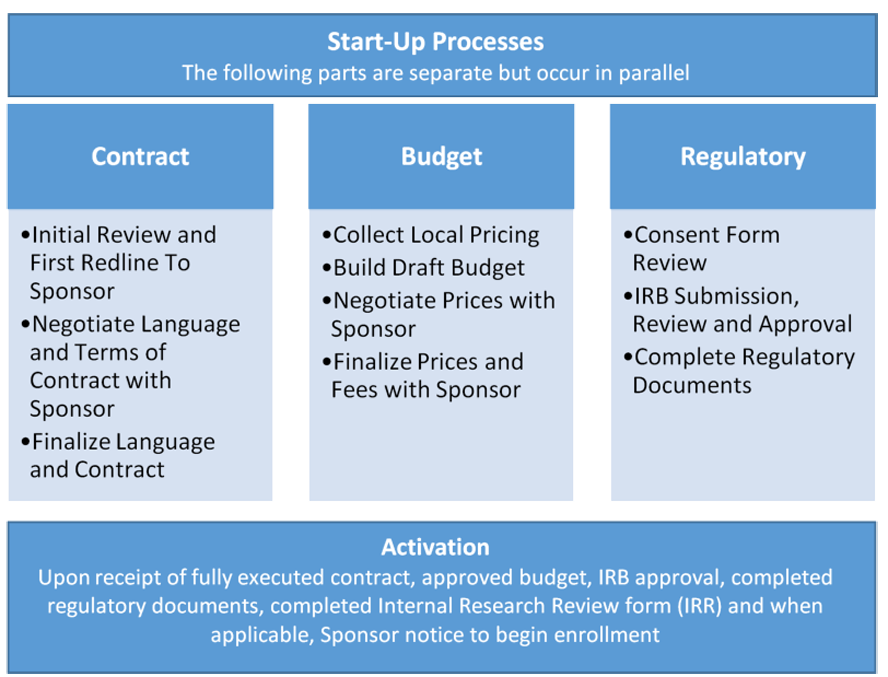
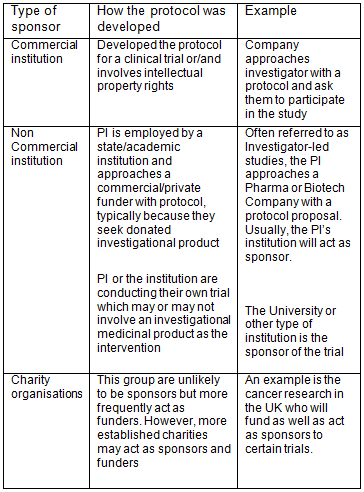
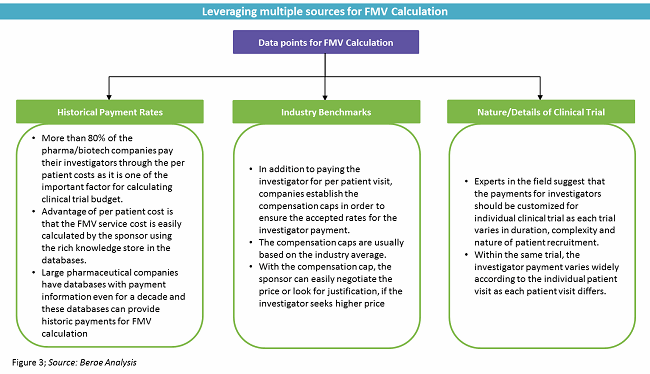
WHEN, WHY AND HOW SPONSOR, CONTRACT RESEARCH ORGANISATION (CROs) AND RESEARCH SITES WORK TOGETHER: – your pharmacy guide

Collaboration between the sponsor, academia, and regulatory agencies... | Download Scientific Diagram

Inside Clinical Trials ® ALL RIGHTS RESERVED. What is a clinical trial? ALL RIGHTS RESERVED. - ppt download