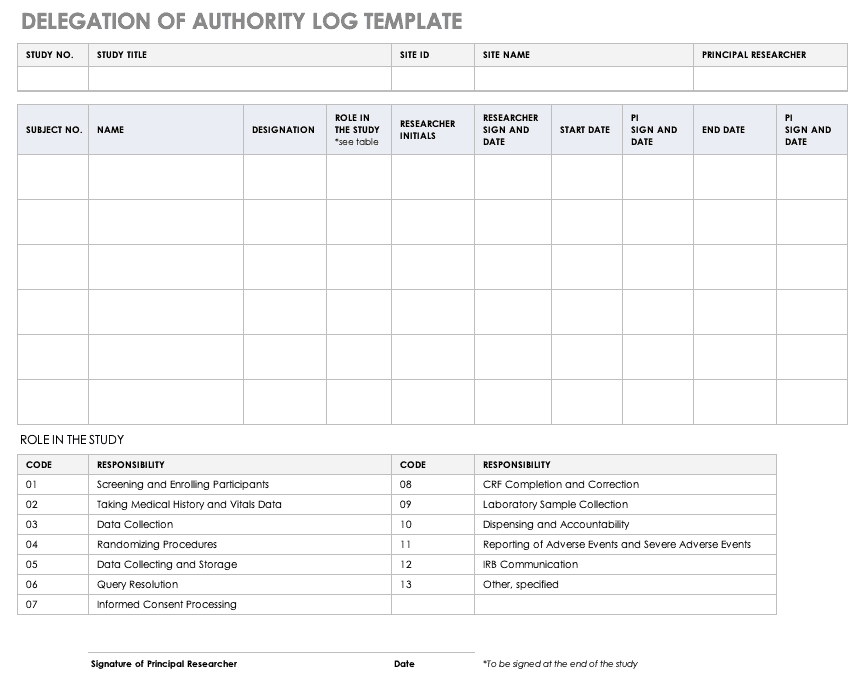
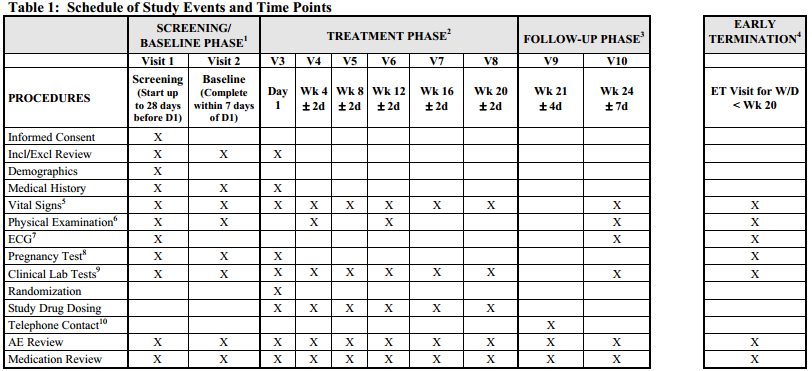
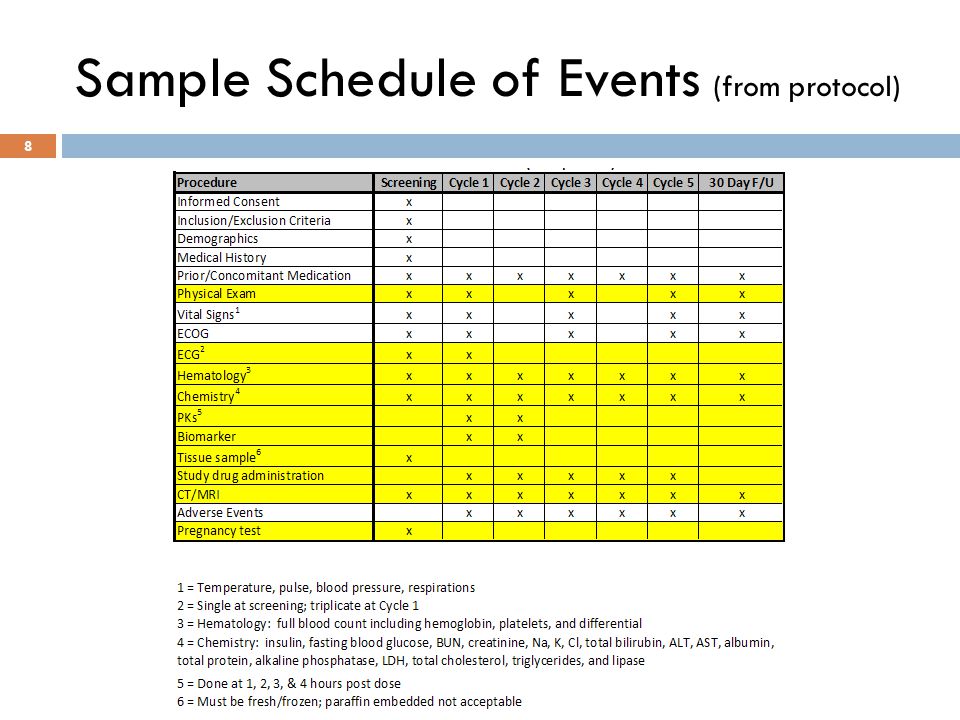
Schedule of Events. Example visit and assessment specification from a... | Download Scientific Diagram

Effectiveness and safety of electroacupuncture for poststroke patients with shoulder pain: study protocol for a double-center, randomized, patient- and assessor-blinded, sham-controlled, parallel, clinical trial | Semantic Scholar

Schedule of Events. Example visit and assessment specification from a... | Download Scientific Diagram
PLOS Medicine: Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis
Therapeutics for Dengue: Recommendations for Design and Conduct of Early-Phase Clinical Trials | PLOS Neglected Tropical Diseases
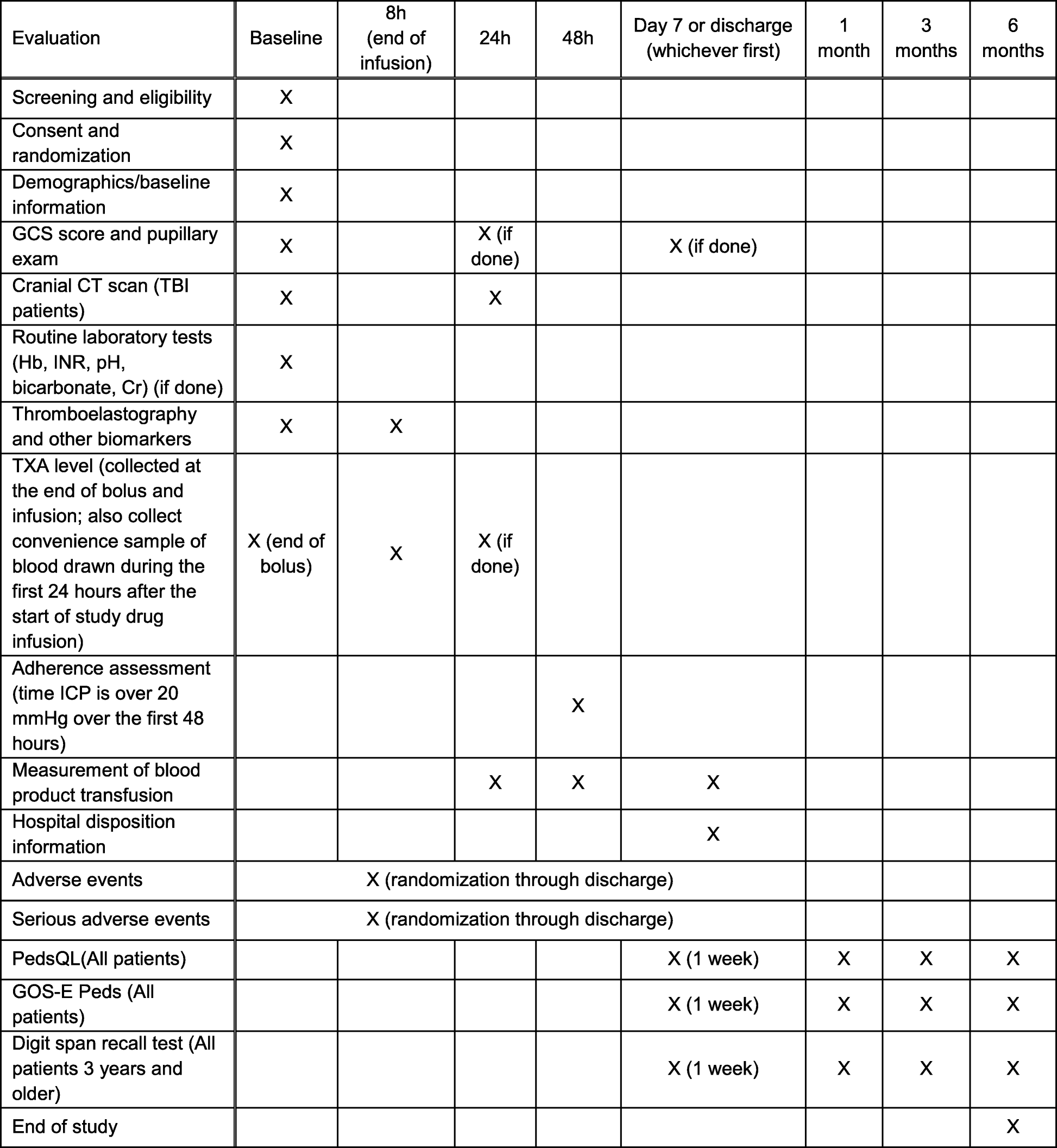
Traumatic injury clinical trial evaluating tranexamic acid in children (TIC-TOC): study protocol for a pilot randomized controlled trial | Trials | Full Text

NHSBT/MRC Clinical Studies Unit Platelets for Neonatal Transfusion Study 2 (PlaNeT-2) A randomised controlled trial of platelet transfusion thresholds. - ppt download

Protocol of the Definition for the Assessment of Time-to-event Endpoints in CANcer trials (DATECAN) project: formal consensus method for the development of guidelines for standardised time-to-event endpoints' definitions in cancer clinical trials.

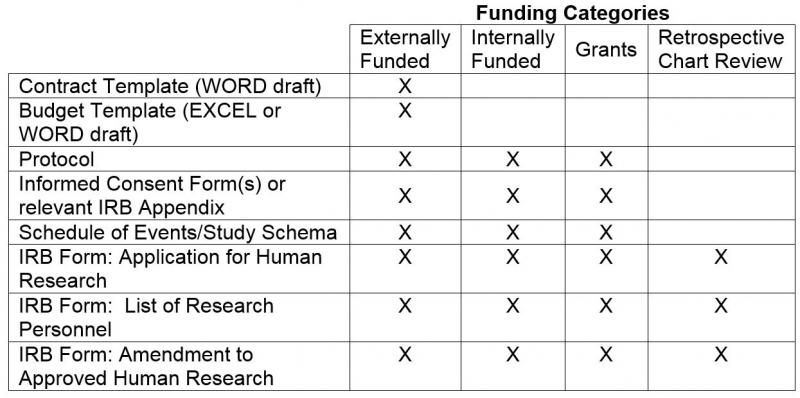
David Cloutier Director, Research Center Management and Development Budgeting for Industry Sponsored Clinical Trials. - ppt download

An analysis of subject recruitment issues for an HCV investigational drug clinical trial | Semantic Scholar
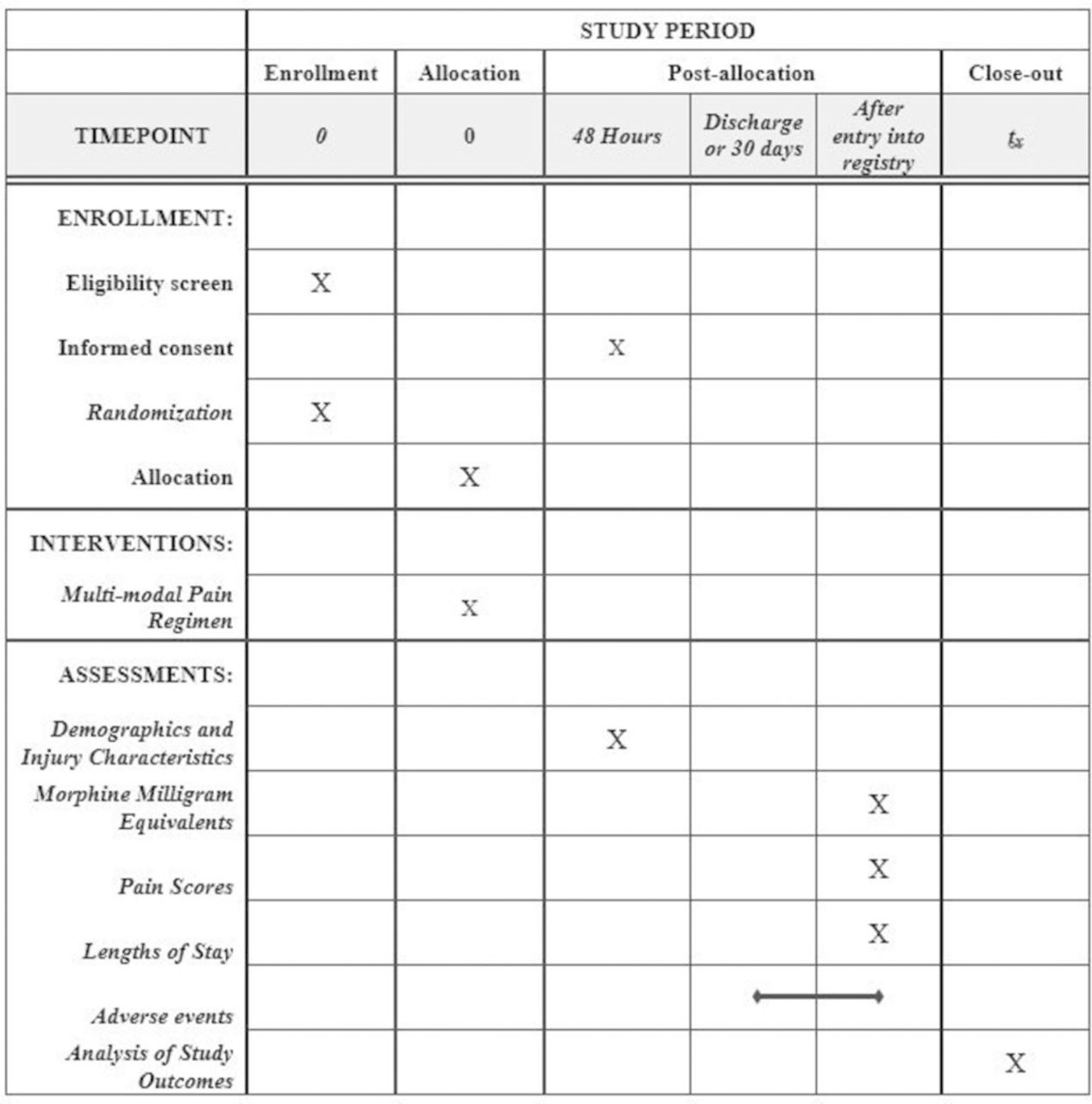
Multi-modal Analgesic Strategies for Trauma (MAST): protocol for a pragmatic randomized trial | Trauma Surgery & Acute Care Open