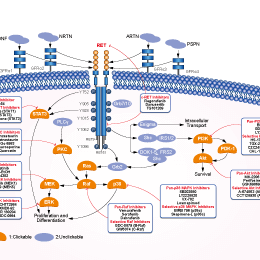
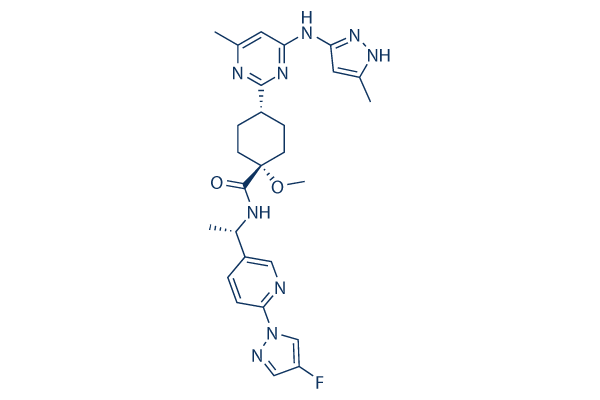
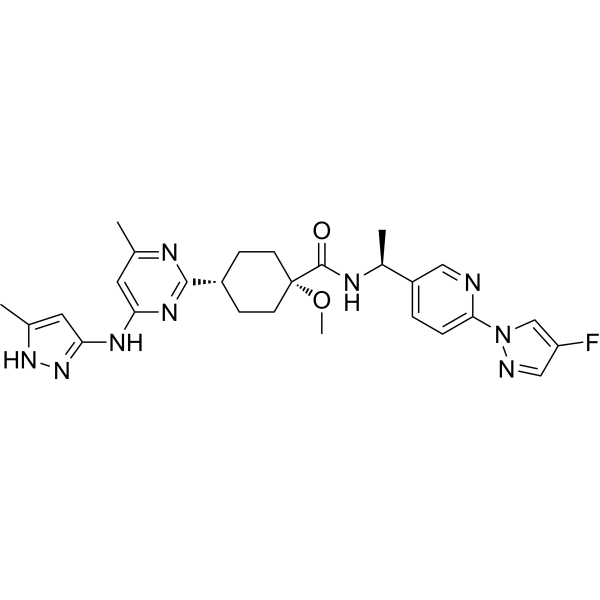
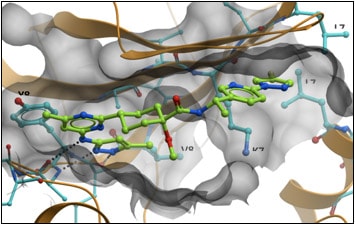
New Biomarkers - NSCLC Treatment Paradigm - Text Module - NSCLC Practical Guidance - Oncology - Clinical Care Options
Clinical activity of the RET inhibitor pralsetinib (BLU-667) in patients with RET fusion+ solid tumors

ARROW: BLU-667 for RET+ NSCLC - Capsule Summary Slidesets - Lung Cancer - 2019 ASCO Annual Meeting - Oncology - Clinical Care Options

RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies - Journal of Thoracic Oncology
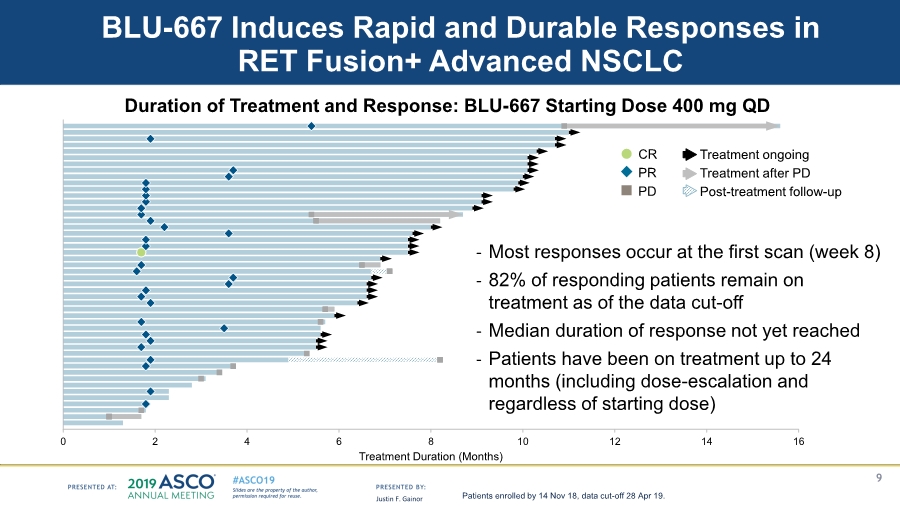
EX-99.5 6 ex-99d5.htm EX-99.5 Exhibit 99.5 GRAPHIC Clinical Activity and Tolerability of BLU-667, a Highly Potent and Selective RET Inhibitor, in Patients with Advanced RET-Fusion+ Non-small Cell Lung Cancer Justin F. Gainor1, Dae Ho Lee2, Giuseppe ...
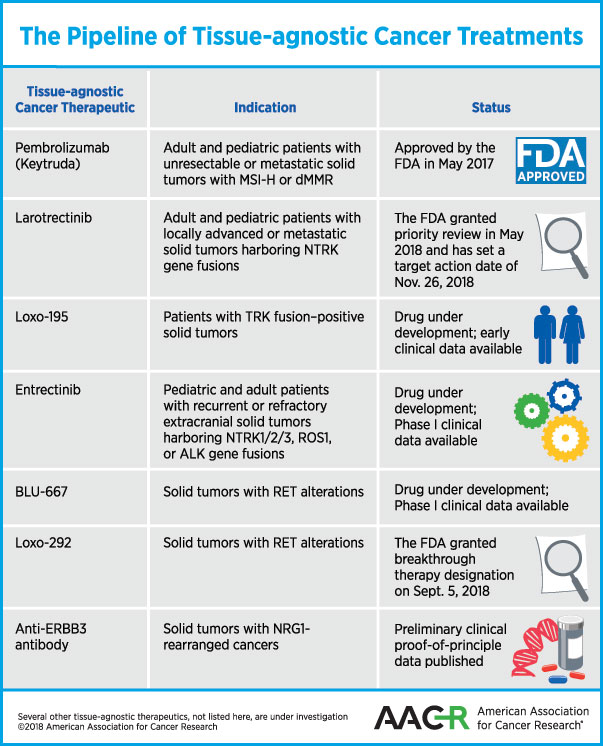
Harnessing the Power of Precision Medicine – Treating Cancers with Tissue-agnostic Therapies - American Association for Cancer Research (AACR)